“Divers who suffer decompression sickness have a patent foramen ovale (PFO) prevalence twice that of the population in general.”
Having healthy heart valves is essential if your heart is to properly pump and circulate blood throughout your body. Some people are born with structural anomalies in their heart valves or in the walls. Many such disorders are diagnosed early in life and corrected, restoring the affected individuals’ exercise capacity and enabling them to dive safely. However, some inborn structural disorders, like a condition known as patent foramen ovale, may not become obvious until after an affected individual has taken up diving — and may result in an increased risk of certain diving injuries. In addition, some people are impacted later in life by acquired valvular damage that may affect their fitness to dive.
In this chapter, you learn about:
Overview of Valvular Disorders
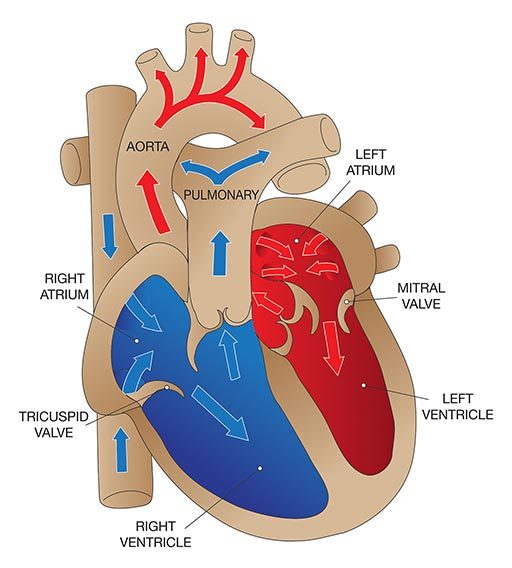
The heart has four main valves that facilitate the pumping activity of the heart:
- The tricuspid valve, between the right atrium and the right ventricle.
- The pulmonary valve, between the right ventricle and the pulmonary artery.
- The mitral valve, between the left atrium and the left ventricle.
- The aortic valve, between the left ventricle and the aorta.
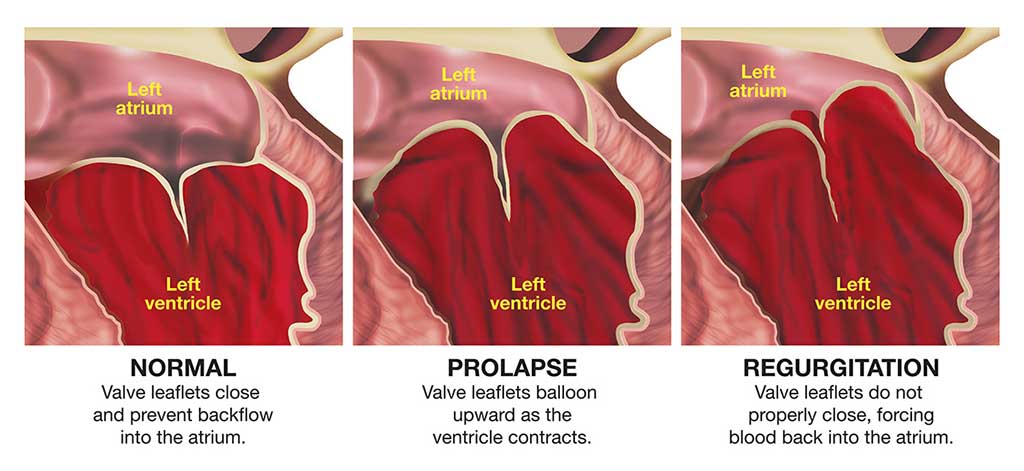
Each valve consists of a set of flaps (also called “leaflets” or “cusps”) that open and close to enable blood to flow in the correct direction. The function of the valves may be compromised by either congenital or acquired abnormalities. Damage to the valves can occur due to infection, rheumatic fever or aging. For example, the opening in a valve may narrow (a condition known as “stenosis”), meaning the heart has to work harder to get blood through the opening; this generates higher pressure within the heart and eventually causes the cardiac muscle to overdevelop. Another common valvular problem is incomplete closure, which allows the blood to flow backward through the valve (a condition known as “regurgitation”); this overloads the heart with blood, eventually resulting in enlargement (or “dilatation”) of the heart’s cavities.
The two most common valvular disorders in older adults are aortic stenosis and mitral regurgitation. The symptoms of valvular disorders vary depending on which valve is affected as well as on the type and severity of the change. Mild changes may cause no symptoms; a heart murmur — detected when the heart is examined with a stethoscope — is often the first sign of valve damage. In aortic stenosis, however, exertion can cause chest pain (known as “angina”) or a feeling of tightness in the chest, shortness of breath, fainting or heart palpitations. Sudden death in otherwise healthy athletes is sometimes caused by aortic stenosis. Regurgitation can also cause detectable symptoms, such as shortness of breath or wheezing when lying down; these complaints may be intensified by exercise, increased resistance to breathing and immersion.
Treatment for valvular disorders generally involves surgery. Defective valves may be either repaired or replaced by prosthetic valves.
Preventing valvular damage is, of course, the best approach. Routine physical exams may uncover evidence of early valvular disease. In such cases, close, regular medical surveillance is advised to identify, and hopefully slow, progression of the damage.
Effect on Diving
Significant valvular anomalies may preclude diving until they can be corrected. Even after corrective surgery, there must be an assessment of such factors as exercise capacity, the presence of any residual regurgitation and the need for anticoagulation. Such an assessment should include a detailed examination of the heart and of the individual’s ability to exercise at a level consistent with diving, without evidence of ischemia, wheezing, cardiac dysfunction or a problem known as “right-to-left shunting.”
Mitral Valve Prolapse
Mitral valve prolapse (MVP) may also be referred to as “click-murmur syndrome” or “floppy-valve syndrome.” It is a common condition, especially in women. The problem arises as a result of excess tissue and loose connective tissue in the heart’s mitral valve, so that part of the valve protrudes down into the left ventricle during each contraction of the heart.
An individual with MVP may have absolutely no symptoms or may exhibit symptoms ranging from occasional palpitations or an unusual feeling in the chest when the heart beats, to chest pain or a myocardial infarction (or heart attack). MVP is also associated with a slightly increased risk of small strokes (known as “transient ischemic attacks”) or a transient loss of consciousness.
Beta blockers — drugs commonly used to treat high blood pressure — are occasionally prescribed for mitral valve prolapse. They often cause a drop in maximum exercise capacity and may also affect the airways. These side effects normally pose no problem for the average diver, but they may be significant in emergency situations.
Effect on Diving
Frequently, MVP results in no changes in blood flow that would prevent an individual from diving safely. A diver with MVP who has no symptoms (that is, no chest pain, alteration in consciousness, palpitations or abnormal heartbeats) and who takes no medication for the problem should be able to safely participate in diving. But anyone with MVP who exhibits an abnormal cardiac rhythm, which can produce palpitations, should not dive unless the palpitations can be controlled with a low dose of antiarrhythmic medication.
Patent Foramen Ovale
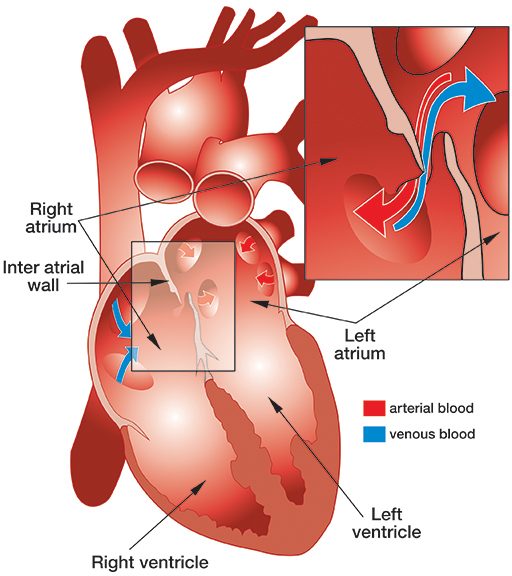
Patent foramen ovale (PFO) is a fairly common, congenital, generally benign hole between the heart’s left and right atria (see illustration).
While a fetus is developing in utero, the wall separating the left and right atria of the heart develops from the septum primum, which grows up, and septum secundum, which grows down. The septa overlap, creating a sort of trap door (known as the “foramen ovale”), which allows oxygenated blood from the mother’s placenta that has entered the fetus’ right atrium to pass through to its left atrium. At birth, the baby’s lungs expand, and the resulting pressure in the left atrium closes the foramen ovale. Typically, shortly after birth, this former opening fuses shut — but in about 27 percent of babies, it fails to fuse completely and results in a PFO.
A PFO often causes no symptoms, and most people who have one are never aware of the fact. PFO is diagnosed by injecting a small amount of air into a vein and observing its passage through the heart using echocardiography. There are two methods of echocardiography. Transthoracic echocardiography (TTE) is easy and noninvasive — it involves simply placing an ultrasound probe on the outer wall of chest — but it detects a PFO in only 10 percent to 18 percent of the population — about half of those who probably have one. Transesophageal echocardiography (TEE) — which involves local anesthesia and intravenous sedation, so the probe can be passed into the esophagus — detects a PFO in 18 percent to 33 percent of the population. However, even though TEE is more sensitive than TTE, there are still many false-negative results with both techniques; a properly conducted TTE may in fact be more reliable than a TEE.
One of the most common treatments for PFO is a procedure called transcatheter closure; it involves threading a catheter through the groin and up the femoral vein into the heart, where a device called an occluder is implanted across the PFO. Occluders come in various shapes and forms, but most act like a double umbrella that opens on each side of the atrial wall and seals the hole. With time, tissue grows over the occluder and completely covers its surface. The implantation is performed under local anesthesia and intravenous sedation, and the patient remains conscious. It takes less than an hour and can be performed on an outpatient or one-night-stay basis. Most patients can return to their normal activities in two days, but they must take anticoagulant and/or antiplatelet drugs for three to six months. Other postoperative restrictions include no elective dental care (such as cleanings) for three months, no contact sports for three months and no heavy lifting for one week. A diver who undergoes a transcatheter PFO closure must abstain from diving for three to six months.
No data is available on the outcome of PFO closure in divers. But the following outcomes were recorded in patients who underwent PFO closure for the prevention of stroke (note, however, that these patients have underlying medical conditions that may contribute to a greater than average risk of adverse outcomes):
- Efficacy: Complete closure of the opening was achieved in 95 percent of cases and incomplete closure in 4 to 5 percent of cases; no improvement was shown in only 1 percent of cases.
- Complications: Overall mortality was less than 1/10th of 1 percent (0.093 percent). The need for a follow-up operation due to an adverse event associated with the device was less than 1 percent (0.83 percent).
- Serious complications: The incidence of death, stroke, infection, bleeding or blood vessel injury was 0.2 percent; of device movement or dislodgement, 0.25 percent; of clot formation on the device, 0.3 percent; of major complications in the perioperative period, 1.2 percent; and of minor midterm complications, 2.4 percent.
Effect on Diving
Divers who suffer decompression sickness (DCS) have a PFO prevalence twice that of the population in general. And in divers who exhibit neurological DCS symptoms, PFO prevalence is four times greater. The risk of DCS seems to increase with the size of the PFO. Based on these facts, it is assumed that divers with a PFO are at greater risk of DCS than those without a PFO; however, the only prospective study designed to directly measure the relative risk of DCS in divers with a PFO is still ongoing.