We live in the Anthropocene: Humans affect the very world we inhabit. Deforestation, fossil fuel use and environmental pollutants such as plastics and pesticides are changing our world. The oceans and the reefs within them are not immune to these effects.
Coral-bleaching events and the coral death that often follows are among the most serious challenges in marine ecology today. Despite decades of scientific investigation, researchers have not yet discovered an effective way to identify the onset of bleaching events, which result in the loss of the endosymbiotic algae that reside within corals. The algae contain chlorophyll and use photosynthesis to produce carbohydrates that they supply to the corals. Without the algae, there’s no chlorophyll for the corals.
Researchers can monitor changes in terrestrial chlorophyll by examining near-infrared chlorophyll fluorescence (NIRChlF), even by satellite, but they have done little work on near-infrared (NIR) imaging underwater. The first 6 feet of the ocean’s surface absorbs most NIR light from the sun, so aerial or satellite imaging of NIRChlF isn’t possible.
We recently published a scientific paper using commercially available equipment to image NIR fluorescence from chlorophyll in corals.1 We lack the time and ocean access, however, to do much of the basic control work that might allow remote underwater imaging of NIRChlF to become a part of the solution to identifying bleaching events. We invite divers and underwater photographers who are willing to make a modest investment in their imaging equipment to help us further develop and improve our methodology. Any diver can become a part of the solution by engaging in citizen-science research.
We affectionately call the camera setup we used to get data for our published work “the beast.” Not all the equipment is necessary to begin, but an understanding of how we have assembled our NIR fluorescence-imaging system should help others to mimic and improve upon it.
You must have a camera with custom white-balance settings and an underwater housing. You will need to remove the internal infrared filter, which you can do yourself if you are a camera expert or you can pay a commercial dealer about $250 to make the modification for you. You will also need a longpass NIR filter (about $15) to put over the lens.
If you want to get more serious, a pair of good tricolor or blue LED dive lights ($150–$1,000), hardware and tripod ($100–$400), shortpass light filters ($250) and a dual diopter holder for the filters ($250) will allow you to make your own similar beast setup.
To illustrate the process, we collected images of a great star coral (Montastraea cavernosa) on a night dive in the Caribbean Sea near Carriacou, Grenada. We used a camera with a macro lens on a tripod to take these images, but neither the tripod nor macro lens is an absolute necessity. For the far-left image we used white-light illumination and no filter. The middle image is with blue-light illumination and a yellow filter for viewing green fluorescent proteins and red emission (mostly from chlorophyll). The right image is with blue LED or white illumination below 675 nanometers (nm) and an IR 720 nm filter on the camera showing only the NIRChlF.
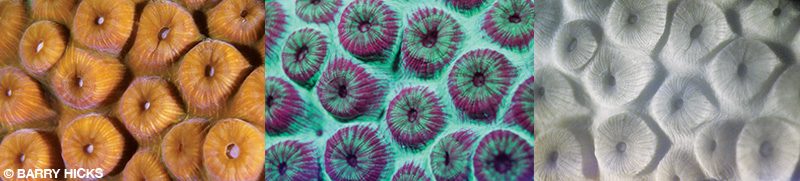
The center image clearly shows that fluorescent proteins and chlorophyll are not identically distributed, which may explain why fluorescent proteins don’t prevent solar-induced bleaching in the native reefs despite research indicating that they can act as a sunscreen. If this sunscreen is supposed to help protect the endosymbiotic algae inside the corals, it is not sufficiently applied to do the job. The NIRChlF image on the right confirms the chlorophyll distribution. Regions in the center image showing the most intense red fluorescence are also the brightest in the NIRChF image.
Divers can perform this type of fluorescence imaging in daylight because of limited infrared light penetration below about 10 feet. To start your own imaging sequence, find a coral near a sandy bottom where the tripod can be set up to minimize impact. Use a white dive slate as a target for setting custom white balances on the camera; generate a separate setting to use each with each combination of lighting and filter set (white lighting with no filter, blue LED lighting with yellow filter, and blue lighting with a NIR 720 nm longpass filter). Using a tripod will ensure that all the images will be in register.
A standard lens at a greater distance rather than a macro lens might capture more information on larger specimens and without a narrow depth of focus, but it could require longer exposures and necessitate use of a tripod to prevent blurred images. A macro lens is desirable because it produces a greater level of fine structure on chlorophyll distributions, and the close working distance between camera and subject means the water absorbs less NIR light. As with all macro photography, issues such as narrow focal depth can be problematic, but once you get started you’ll be hooked.
We want to improve this imaging methodology so autonomous underwater vehicles (AUVs) can use it for remote imaging on large sections of the reef, similar to satellite imaging for terrestrial NIRChlF. While there are no guarantees, such technology might be able to identify the onset of a bleaching event.
Besides the unique photos you get from underwater NIRChlF imaging, NIR fluorescence-imaging equipment can help with other research. With assistance from the dive community, we would like to investigate the following questions:
- How deep can we photograph NIRChlF emission in daylight without external illumination?
- How deep can we find organisms that have endosymbionts containing chlorophyll?
- How far from specimens can we photograph NIRChlF emission underwater with either ambient solar illumination (in daylight) or external LED illumination (at night)?
- What lenses work best at the maximum distance?
- Which sponges have algal endosymbionts?
- What unexpected marine organisms (such as tunicates or bivalves) have chlorophyll-containing endosymbionts?
- Which organisms that normally contain chlorophyll (such as gorgonians) have members that do not?
- How much does chlorophyll content change seasonally in one coral?
- How much NIR does endolithic algae in bleached or diseased corals emit?
- Using video and time-lapse analysis, does chlorophyll redistribute when illuminated? Can this be detected? On what time scale? How does this vary with species?
- Can chlorophyll photobleaching in one polyp be overcome by donation of chlorophyll from a neighboring polyp?
- Art can motivate some people in a way that science cannot. Altering white balance, lighting and filters when performing underwater NIR fluorescence imaging can lead to color effects like those seen in terrestrial NIR images. What types of art can you produce to motivate others to pursue underwater NIR fluorescence imaging?
There clearly is much to be done. If you are interested in helping to conduct some of these experiments and would like more information, contact Barry Hicks at .
Reference
1. Oh T, Sermsripong J, Hicks BW. Using scuba for in situ determination of chlorophyll distributions in corals by underwater near-infrared fluorescence imaging. J. Mar. Sci. Eng. 2020; 8(1):53.
© Alert Diver — Q3/Q4 2020