Divers, who are inquisitive by nature, are always looking for new ways to experience the wonders of the underwater world. One increasingly popular diving technique, called fluorescence (fluo) night diving, gives divers the ability to observe marine creatures in brilliant, glowing colors invisible to the naked eye.
Fluo diving relies on the property of some marine life to emit longer wavelengths of visible light when illuminated with shorter-wavelength blue light. The term “emission” is very important to understanding the physics of fluorescence. The emission of light differs from the reflection of light that happens when, for example, you take your white light torch on a night dive. In traditional night diving, white light is reflected off of the reef or organism and bounced back to your eyes. Emission light, however, is light that the organism creates and emits back to you. The process is similar to bioluminescence in that the organism creates its own light; however, in bioluminescence the light, which is generated by chemical reaction, requires no excitation light.
To view biofluorescence, fluo divers equip themselves with blue-light torches and barrier filters for their masks (and cameras, if they are doing photography). The barrier filter’s function is to block the blue light that is reflected back to the observer from the organisms on which the light is shining. All that would be visible without the barrier filter is a very bright blue light, but the filter is designed to cut off all or most of the wavelengths in the blue part of the spectrum. The intensity of the emission light from the organism is very dim — so dim, in fact, that it is completely overwhelmed by the blue light; but if you block the blue, all you will see are the emission colors.

The wavelength of light used in most fluo torches is a narrow band of blue, somewhere between 440-480 nanometers (depending on the manufacturer). Fluo diving differs from ultraviolet (black-light) diving because UV light is in the sub-400nm range. Some companies produce UV torches for underwater use because invisible UV excitation light has the advantage of requiring no additional filters, but researchers have also discovered that blue light is more efficient in stimulating green fluorescent protein (GFP) and its mutations, which emit colors other than green.
Blue light is so effective because (as we all know from our beginner open-water course) it is the only light available at depths beyond about 30 feet, which means that this is the light in which organisms such as coral have evolved over the eons. Most UV light from the sun bounces off the surface of the water, and the light that penetrates makes it only a few inches, rendering UV light an inefficient light source for fluo diving.

Not all marine organisms exhibit the fluo effect, but for those that do the visual demonstration can be dramatic. Some examples of fluorescing species include anemones, a variety of shelled animals, some types of fish, coral polyps and both soft and hard coral structures. The terms “hard” and “soft” coral can be a bit misleading. For example, brain coral is often mistaken as hard coral, but it is considered to be in the long polyp stony (LPS) family of soft coral. The small polyp stony (SPS) family of coral is similarly misrecognized as hard coral even though it is actually soft. These misidentifications are due to the fact that, in both cases, the living coral is made up of tiny soft creatures that live and die building up large stony structures over the course of decades. Interestingly, these two species are the coral subjects that emit the most fluo effects. Examples of soft coral that rarely fluoresce are generally in the Alcyonacea order; it is important to note, however, that in all groups there are exceptions to the rule — just like with people.
The basic point is that there is great variance in the types of coral that fluoresce, and we have not yet determined all of the rules of this phenomenon. This is one of the allures of fluo diving: You can make your own discoveries as a citizen scientist.
Many people think that fluo diving is done only for views of the spectacular colors or for the purposes of underwater photography. It certainly meets those expectations and can indeed be a life-changing experience, but it is also much more than that. Fluo diving has become an indispensable tool for coral-health research efforts and coral-propagation census analysis. If you come upon a polyp or drifting coral larvae with white light, you will see little or nothing; with the proper fluo diving gear, however, the individual, almost-microscopic organisms will shine in the sand like sparkles in the snow on a moonlit night. Not only is this amazing to witness, but it also provides scientifically valuable data.

Coral reefs are considered the rainforests of the ocean. In normal waters, corals develop a symbiosis with single-celled algae called zooxanthellae, which use photosynthesis to provide food and energy to the coral. When water temperatures rise, the zooxanthellae are ejected, removing the vital nutrients the coral needs to survive and causing “bleaching” of the coral. The coral bleaching that accompanies rising temperatures makes the coral vulnerable to additional stresses that can ultimately destroy the entire reef. Apart from coral bleaching, ocean acidification reacts with the coral’s calcium-carbonate skeleton, causing it to break down and dissolve. These effects can be witnessed under white-light conditions, but they are even more dramatic when using fluorescent technologies.
Increasing numbers of marine institutes and universities are using fluo equipment to assess the effects of rising ocean temperatures and acidification on coral in addition to other more general coral studies. Fluo diving has even led to discoveries of previously unknown species that were too small to see with white light but shine like beacons in the dark when illuminated with blue light.
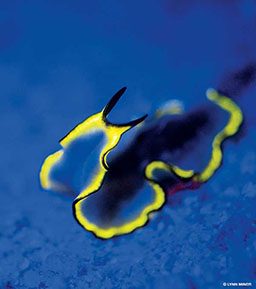
It is not well understood why some corals and other sea creatures evolved to fluoresce, but what is known is that some marine organisms — including corals, tunicates, barnacles, sponges, anemones, jellyfish, clams, nudibranchs, cephalopods, shrimp, crabs, worms and fish — produce GFP and mutations of GFP that react when illuminated with a blue light. The vast variety of species that demonstrate this effect suggests that fluorescence is not simply an accidental byproduct of some other evolutionary function but likely serves some currently unknown purpose.
Theories abound as to why these species evolved to fluoresce. One thought is that fluorescence serves as a form of sunblock that can protect corals and other species in shallow water from UV energy; other theories posit fluorescence as a means of intraspecies communication. The evolutionary biology of fluorescence constitutes a thriving area of study in many marine institutes and universities.
Still, fluo diving is not only for scientists. If you would like to try fluo diving, the Professional Association of Diving Instructors (PADI) has a Fluorescence Night Diver distinctive specialty course that I wrote, which several dozen certified instructors around the world teach. The course covers the science of fluo diving in much greater detail and specifically emphasizes the safety implications unique to fluo diving.
If you decide to fluo dive, remember that almost no light remains when you put on your blue barrier filter; the emission light you see is dim and cannot light up the entire reef, and the filter eliminates your only other light source. Therefore, you must exercise excellent buoyancy control and remain constantly aware of your surroundings. If a coral head doesn’t fluoresce, you can crash into it, so you should always approach and depart a site using your backup white-light torch and have it handy when you enter an area with little fluo activity. Alternatively, you can always remove your mask filter and see fine — in blue.
The Physics of Fluorescence
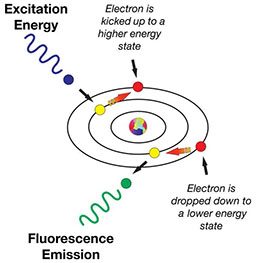
The schematic representation of the fluorescence effect demonstrates the mechanism when blue light strikes the protein GFP, which absorbs the blue light’s energy. The absorption of the light’s energy causes the electrons of the protein’s constituent atoms to make a quantum jump from one valence electron shell to a higher shell. The change of energy state then instantly “decays” back to its quiescent resting state. When the decay occurs, the electron gives up, or emits, a photon of light, but at a lower energy and longer wavelength.
In summary, when you illuminate the protein with blue light, it emits back in other colors of the spectrum, including green, yellow, orange and red. The color emitted is determined by how many jumps the electron makes.
© Alert Diver — Q4 Fall 2014